For small, mobile medical devices, designers are discovering a ready-made user interface and connectivity technology in the Apple iPhone, which can run apps to present data from medical devices and deliver that data where needed.
Cell phones are undoubtedly the most ubiquitous handheld platform in the world, and have become attractive deployment platform candidates for medical devices. It’s no wonder then that software application developers as well as traditional medical device manufacturers are recognizing the tremendous potential for smart phones as an alternative to custom embedded devices and custom user interfaces for their new medical devices. In addition, the use of these wireless devices is enabling a new level of medical device connectivity. And when it comes to smart phones and medicine, it seems that Apple’s iPhone is leading the revolution.
A recent survey by Manhattan Research “Taking the Pulse U.S. 11.0,” an annual report that examines how physicians are using technology, found that 75 percent of physicians in the United States have purchased an Apple mobile device and that the iPhone was their favored smart phone platform. However, with the use of these mobile platforms comes unknown variables, not the least of which is FDA regulation. We surveyed a variety of manufacturers who have come to market with medical devices based on the iPhone, and asked them to share what made them choose this platform over other smart phone alternatives.
AirStrip Technologies, a medical software development company focused on mobility in healthcare, specializes in “space-shifting” real-time and historical medical data that is intended to be visualized. To allow them to rapidly develop and deploy native mobility applications, AirStrip has built a custom technology platform, AppPoint, designed to take raw data from bedside medical monitoring devices and “airstrip” this data up to the Cloud, to be subsequently rendered on a native device such as an iPhone. The AppPoint platform and products deployed on it eliminate the physical and geographical boundaries of medical information, getting data to people when and where they need it.
AirStrip’s initial application built on AppPoint was AirStrip OB, used for remote labor and delivery monitoring (Figure 1). Since a woman is often in labor for a period of time, the attending physician cannot be with her constantly to monitor her progress. AirStrip saw the opportunity to employ smart phones as a means to extend the presence of medical devices from patient bedside to wherever the physician might be. With OB, the physician can see exactly what the attending nurses are seeing at the patient bedside, even with the weakest of cell signals.
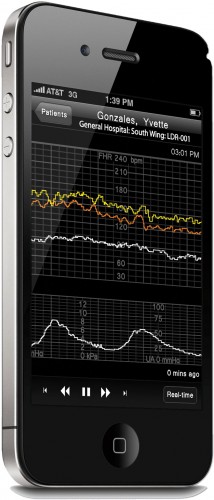
Figure 1: AirStrip OB delivers vital patient waveform data— including fetal heartbeat and maternal contraction patterns—in virtual real time directly from the hospital labor and delivery unit to a medical professional’s mobile wireless device.
In bringing OB to market, AirStrip went through a process of getting their proprietary mobility platform and OB classified as a type II medical device. Bruce Brandes, an executive vice president and chief strategy officer for AirStrip, reports that although they were not instructed by the FDA to obtain clearance for their mobility platform and products, AirStrip chose to undergo certification anyway since their applications support remote, real-time diagnostic capabilities for clinicians. Once they proved the concept, AirStrip started to build other applications on AppPoint such as AirStrip Cardiology for EKG management, now on the market, and AirStrip Patient Monitoring, now in development. Brandes comments that “Each new application requires a new FDA clearance, but the fact that we build our products on the previously certified AppPoint engine results in faster and easier preparation for clearance of these new products.”
While Airstrip focuses on creating products for physicians that interact with hospital-based medical devices, Withings is creating products for patients to use in their homes or on the go. In setting out to build its medical products, Withings chose a phone for a platform, the iPhone in particular, as a way to use a familiar interface to interact with rich information. This essentially turns the iPhone into the user interface for a medical device such as Withings’ blood pressure cuff, which just received FDA clearance (Figure 2).

Figure 2: The Withings blood pressure monitor makes taking your blood pressure as easy and straightforward as making a phone call.
Cedric Hutchings, Withings’ founder and general manager, describes why they selected the iPhone as a deployment platform. “The iPhone sets a reference for simplicity to the user. Simplicity is the key and the most important point if a manufacturer wants a new device to be adopted by the consumer and to be used in the long term.” In fact, partnering with Apple in the ‘Made for iPhones (MFI)’ device program allows Withings to provide a very simple and consumer-friendly interface. “It couldn’t be simpler,” explains Hutchings. “The user simply plugs a monitoring device into the iPhone and automatically the right app is launched. All the user has to do is push start to begin the measurement.”
On the topic of whether the iPhone platform has advantages over other smart phones for the development and deploy-ment of medical products, Hutchings noted that Apple is managing a program for device compatibility and this enables certain “features” that are not in other platforms yet. “The iPhone is not as open a platform for third-party development (compared to other smart phone platforms), which is not always a good thing for product developers such as us. But the good thing is if a manufacturer is compliant with this architecture and compliant with the iOS and device, you are provided with a more regulated and hence stable and predictable platform on which to develop and deploy your medical device.”
Hutchings is convinced the iPhone in particular will enable a new generation of devices as it allows for the creation of “connected” rather than “connectable” devices. “You can create connectable devices, for instance where you take a regular blood pressure monitor and add connectivity like Bluetooth,” states Hutchings. “But with current technical constraints, this is not a very user friendly end to end solution and you end up having a more complex device than the non-connected version.” Hutchings goes on to say, “In this scenario the user needs to pair the pressure cuff with a device, needs to have software on their device to talk to the cuff, and then needs to do something with the measured data, and so on. With a connected device, the app takes care of the user experience. Connectivity will enable a more simple experience, and this will enable a revolution.” In support of this view, Hutchings offers that the Withings pressure cuff is not a piece of the hospital that sits at home. Rather it is consumer-centric, something that empowers consumers to be involved in their own health. And since both the Withings blood pressure cuff and scale are distributed in the Apple store, this selling of health devices by Apple demonstrates the breaking wave of consumer adoption of connectable health devices.
Large traditional medical device companies are also seeing opportunity in developing for iOS devices. Take Andon Health, for instance, one of top three blood pressure manufacturers in the world. Adam Lin, the SVP and general manager for iHealth, states iHealth was formed last year by Andon. As Lin recounts, “We looked at what Apple was doing in the iOS space and we had a vision for a new class of iOS-based peripheral products. We then made a critical decision to form a new company to develop and market these devices, and our first product is the BP3 blood pressure monitor.” Lin goes on to state that the iHealth products are also currently being offered to customers in the Apple Stores, and will be expanding to big box stores in the fourth quarter of this year as well.
When explaining why iHealth chose to create a product based on the Apple iOS as opposed to another device, Lin explains “we felt that with iOS’s strong penetration and growth rate, the maturity and sophistication of the app development process, and Apple’s commitment to develop the app accessory space, it was the right decision.” Lin also went on to offer that he thinks the real power and functionality of connected medical devices will be in the application and will result in better integration. “Applications have to be intuitive and not add another layer,” states Lin. “It is the integration, not the form factor that will be the limiting and driving factor in bringing data monitoring devices from the hospital into the home.”
FotoFinder Systems GmbH is a maker of digital imaging products, and recently released a unique device that docks with an iPhone to turn it into a handheld digital dermatoscope for performing skin examinations (Figure 3). Valeska Heinrich, marketing manager for FotoFinder explains why they decided to create a product based on the iPhone. “Dermatology imaging systems exist but these are too expensive in rural areas and most parts of the world. However, with smart phones, doctors have handheld devices that can take pictures, so we decided to take advantage of that to create a digital connected dermatoscope.” When asked why they decided to create a medical product based on the iPhone and not another smart phone platform, Heinrich reported that they felt the iPhone had the best camera on the market, so they decided to convert it into a dermatology camera. She also adds that Apple offers quality standards for its applications unmatched by other manufacturers. “Android is wide open and there are numerous different devices based on this OS. This would require us to develop, test and support a new optic interface for each unique device. Also, the iPhone is popular and widespread among doctors!”

Figure 3: Slip your iPhone into FotoFinder’s handyscope and instantly have a digital dermatoscope for mobile skin examinations.
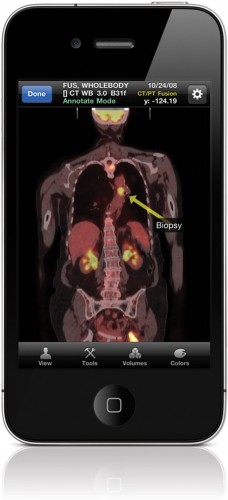
Figure 4: Mobile MIM is a remote diagnostic imaging tool specifically designed for use by medical professionals for remote viewing of medical imagery.
MIM Software, a provider of medical imaging software, recently received a 510(k) clearance for its iPhone-based Mobile MIM, an application for the remote diagnostic viewing of CT, PET, MRI and SPECT images. To protect against instances where Mobile MIM is installed and run on a new iPhone model that has not been tested, Mark Cain, the CTO of MIM Software, states that their application includes registration that communicates with their servers, and that this communication includes the device model. “Our servers will send a message back to the device if it isn’t one that we have fully verified as capable of performing according to our app’s intended use. A message will be presented to the user with instructions that it is not to be used for diagnosis until the model has been verified. In this way, when Apple releases new hardware, the user is told if we haven’t tested it yet.” Cain points out that manufacturers rarely get new Apple hardware sooner than consumers do, so they can only begin testing when it hits the market. After testing is complete, the registered users will be notified of the results, positive or negative. In the same way that MIM verifies the new hardware models, they also verify iOS upgrades. “When the upgrade happens, if we haven’t yet verified the OS, the registered user will be notified that the verification is pending. Should an OS update cause the app to not perform properly, we can notify the user of the problem,” states Cain.
When asked why MIM is developing for the iPhone as opposed to other smart phone platforms, Cain reports, “The process of getting 510(k) clearance can take some time. The consistency afforded by Apple hardware really helps here, especially if the medical device specifically involves the characteristics of the hardware. In our case, image display for diagnosis is inextricably tied to the quality of the display. Having a smaller variety of models, a consistent manufacturing process, and a device life cycle that is long, we can start a 510(k) process and end it using the same devices. Compare this with Android where the number of devices on the market changes monthly; you can understand the difficulty associated with quality assurance.”
In considering connected medical devices, it is interesting to think about some advice offered by Rick Hampton, wireless communications manager at Partners Healthcare, who is cautious on how the FDA is approaching medical products based on handheld platforms such as the iPhone and other smart phones. “The top thing for folks to understand is that the FDA has long regulated software associated with medical devices,” Hampton states. “Until recently, this software was embedded in and a component of a medical device. As medical devices became smarter and more of a generic computing platform, the software itself has become a medical device.
“With the FDA broadening its regulatory scope for software as a stand-alone medical device, companies really need to look at the medical device regulations to find out if their software product will meet the description and perform any functions of a medical device as defined by the FDA. Compared to developing hardware, developing software can seem much easier and most people do not believe that something so easy to do can result in a medical device. The last thing you want is to find out your product is going to be regulated as a medical device after it gets into the market.” In fact, Hampton recounts that he meets a lot of developers who believe medical devices must be highly sophisticated machines like CT scanners and used only in hospitals. This is absolutely false. Indeed, companies looking to jump onto the smart phone healthcare revolution could become medical device manufacturers and not even be aware of it!
AirStrip Technologies
San Antonio, TX
(210) 805-0444

